SASS® 3010
Particle Extractor for Electret Filters

The SASS® 3010 is used to extract aerosols captured by Research International’s electret filters and transfer them to a small fluid volume. It is the ideal companion product to the SASS 3100 dry air sampler and the filter-based SASS 4100 two-stage collector.
Captured particulates can be difficult to remove because induced dipole fields create a strong holding force and must be neutralized. Once released, particulates must then be removed from within the fibrous filter matrix and collected in a small amount of sample fluid.
These processes are efficiently performed in a matter of one to two minutes using the SASS 3010 Particle Extractor.
Download Datasheet Request a Quote
Streamline your Sample Preparation
Ease of Operation
The SASS 3010 extracts particles from electret filters quickly and easily with its simple manual operation.
Compact Portability
With its compact design and simple battery-powered operation, the SASS 3010 is perfect for field work.
Compatibility
Perfect for use with our filter-based air samplers, the SASS 3100 or SASS 4100.
- Features
- Applications
- Documents
- Operation
- Parts & Accessories
Features
Product Features
- Fast, simple extraction from electret filters
- Extraction efficiencies are typically in the range of 60 to 80%
- Lightweight and portable
- Runs on two D-cell batteries
- Fits into the SASS 3100 transport case
Applications
Applications
- Pharmaceutical
- Medical facilities
- Public health
- Clean rooms
- Military
- Food processing
- Agriculture
- Indoor air quality
- Environmental
- Homeland security
Documents
Documents
- SASS 3010 Data Sheet
- Safety Data Sheets
SASS 3010 Particle Extraction Kit (PN 7000-162-234-02) (U.S.)
SASS 3010 Particle Extraction Kit (PN 7000-162-234-02) (E.U.)
SASS 3010 Particle Extraction Fluid (PN 7000-162-233) (U.S.)
SASS 3010 Particle Extraction Fluid (PN 7000-162-233) (E.U.)
SASS 3010 Wash Fluid (PN 7000-162-132-XX) (U.S.)
SASS 3010 Wash Fluid (PN 7000-162-132) (U.S./E.U.) - SASS 3100 Filter Comparison Chart
- Candidate Disinfectants for Research International Products
Operation
Operational Details
Using the SASS 3010
Operation of the SASS 3010 is straightforward. The extractor’s cover is first removed and the filter to be extracted is seated in the extractor cover. The cover is then re-attached and 6 cc of extracting buffer is injected from a dropper bottle vial (Part Number 1760-0006-17). The empty vial is then placed in a sample collection station located in the extractor base (This dual-use vial is used to store extracted sample fluid on completion of the extraction protocol).
In the next step, particulates are dislodged from the filter fiber matrix using acoustic energy, a process that takes about 15 seconds. After this has been done, the liquid sample is transferred to the sample vial by manually pressing the extraction pump plunger shown projecting from the top of the extractor in Figure 1. The extractor cap is then removed and the extracted filter assembly discarded. Overall time to process a filter is about 1 minute; if the extractor’s interior needs to be washed before another filter is inserted, total time is still less than 2 minutes.
No external power is used- two “D” batteries power the sonication step. The batteries can be replaced with no tools by removing 4 finger-nuts on the device’s bottom surface. Due to the short sonication time, a long battery life can be expected.
Extraction Efficiency
Extraction efficiencies are typically in the range of 60 to 80%. To test extraction efficiency, a SASS 3000 and several electret filters were used to collect airborne fluorescent polystyrene beads of 1.8 microns diameter. Each filter was operated for a period of 10 minutes. After the collection phase was completed, the filters were mounted in a SASS 3010 and captured beads transferred to 5 ml of extraction buffer using the protocol outlined below. Extraction efficiencies were then determined using fluorometric assay methods.
It was found that an average recovery of 77% was achieved. A second extraction with an additional 5 ml of extraction fluid resulted in recovery of another 17% of the embedded beads, while two more 5 ml extractions resulted in small 4.5% and 1.5% additions to the total number of beads recovered, respectively.
In a second test series designed to study the effect of particle size on extraction efficiency, fluorescent polystyrene beads of 0.9 to15.0 microns diameter were spotted uniformly over either the filter’s inlet (air-side) or outlet (fan-side) face from water-based particle suspensions: Each fluid spot had a volume of 10 ul. For inlet-side spotting, a dilute surfactant solution was used: dilute surfactant assists in wicking injected fluid and suspended particles deep into the hydrophobic filter matrix. For fan-side spotting, particle suspensions in distilled water were used. Distilled water results in poor penetration of the particles and provides a worst-case scenario of particle position within the filter. That is, during extraction the particles must travel through the filter’s entire vertical cross-section.
After the filters had been allowed to air dry, each was extracted with 6 ml of Research International’s extraction solution (Part Number 1760-0006-17). Recovery percentages are shown in Figure 1 for the air-side and fan-side spotting tests, and for the previously described aerosol loading test. From this Figure it can be seen that extraction efficiency for particles lodged on the air side of the filter shows no apparent dependence on particle size. For particles deposited on the fan side of the filter, there is a noticeable improvement in extraction efficiency as particle size increases, until the difference in extraction efficiency becomes insignificant between inlet- and outlet-side deposition, for particles larger than about 10 microns. Note that the aerosol extraction test results with 1.8 micron diameter particles are very similar to results when the particles were spotted onto the filter’s air-side from a surfactant solution.

Taken together, this data implies that the 3010 extractor can be expected to recover about 60% to 80% of the aerosol particles captured by the electret filters used in the SASS 3000 and SASS 4000 dry samplers.
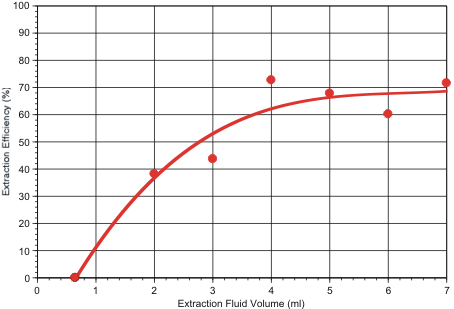
In a third test series, extraction efficiency was determined as a function of extraction fluid volume. For this purpose, 1.8 micron fluorescent polystyrene beads were embedded in the filters by spotting dilute surfactant solutions containing the particles onto the filter’s air-side face, as described previously. Results are shown in Figure 2. These tests indicate that overall collection efficiency is maximized if 4 ml or more of extraction fluid is used.
The comparatively high transfer efficiencies found with a single extraction are the result of several design elements:
- An effective extraction fluid;
- The use of sonic vibration to dislodge particles from the fiber matrix; and
- Regulation of flow so that the extraction fluid enters the rear of the filter and exits the air inlet face, where particle concentrations are highest.
The extraction buffer used by the SASS is compatible with DoD hand-held assays (HHAs) or other lateral flow immunoassays for identification of extracted biologicals. The sample vial may be used to directly apply the sample to an HHA or it may be used directly with other analytical techniques. Please see our Application Note, “Suitability of SASS 2300 Sample Vials and SASS 3010 Sample Vials for use with Hand-Held Assays,” for description of our test procedure that verifies this suitability.
Parts & Accessories
Parts and Accessories
To request a price quote, check the box beside the desired items(s), fill out the contact form, and press Submit.
Please allow two business days for a reply.
Related Products




